In the field of diamond surface coating, after a thorough understanding of diamonds, it will be found that there are rich unsaturated bonds on the surface of diamonds, and four compact tetrahedrons form diamond cells.
For a unit cell, there are three types of carbon atoms:
- One is that all four valence bonds of four carbon atoms are used up, and it is located inside the cell.
- The other is at the center of the six lattice planes of the unit cell, where each carbon atom still has two valence bonds left.
- There are also two types of carbon atoms on the eight top corners of the cell. One is that the carbon atom uses one valence bond, and there are three valence bonds left. There are four such carbon atoms. The other is that there are four valence bonds left, and there are four such carbon atoms.
When a crystal is formed from a unit cell, these valence bonds combine with other units to gradually become a complete crystal, but when they really reach the crystal surface, they become many free valence bonds. Usually, because it is often in a passive state, it is not active, but the passive bond can also be activated, which is the basis for us to use surface theory and surface technology to make good use of diamond properties.
As we all know, the diamond itself is a typical covalent bond crystal. In the crystal structure, each carbon atom combines with four surrounding carbon atoms in a saturated covalent bond to form a regular tetrahedron. Therefore, diamond often shows high chemical inertia. The diamond tools are mainly made by mixing and sintering the diamond and the powder as the bonding agent. Because there is high interface energy between diamond and general metal, resin and ceramic binders, it cannot be infiltrated and bonded by the binder, so there is no interface bonding force between diamond and matrix binder, and diamond is embedded in the binder only by the mechanical wrapping of the matrix. The diamond particles used as abrasives are easy to fall off and run off, leaving a smooth matrix, which makes the tool lose its cutting ability.
Nanodiamond not only has the excellent properties of the diamond but also has the small size effect, surface and interface effect and macro quantum tunneling effect of nanomaterials. It has a very broad application prospect. However, nanodiamond particles have very high specific surface energy, are in a thermodynamically unstable state, and are very easy to spontaneously agglomerate, forming solid aggregates in the synthesis and post-treatment processes. The existence of such aggregates has become an obstacle to development and application. In addition, the oxidation resistance of nanodiamonds is very poor, which seriously hinders the research and development of nanodiamond products.
Diamond surface coating refers to the use of surface treatment technology to coat other materials on the surface of diamond particles to make them have special physical and chemical properties, so as to improve their use effect.
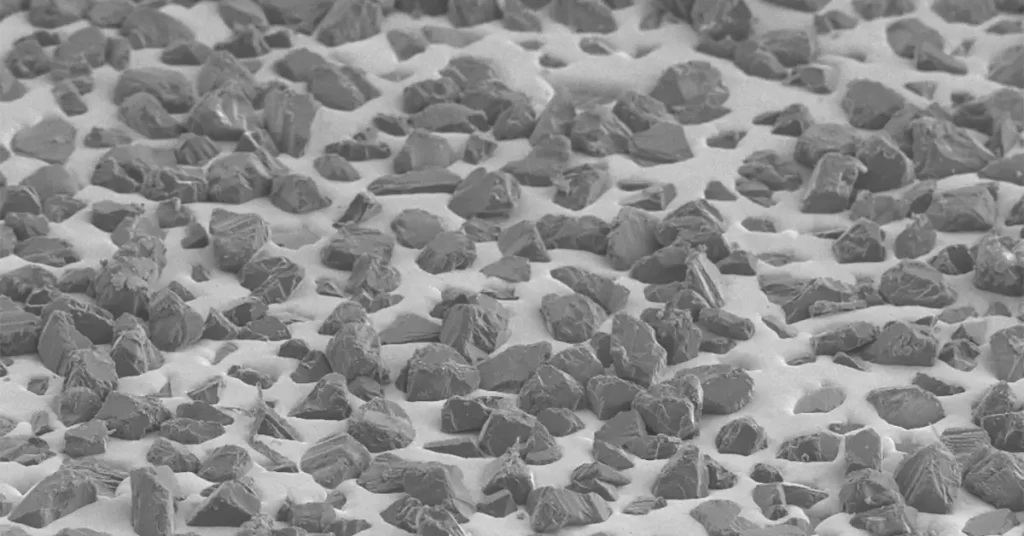
The diamond surface coating mainly has the following functions:
- Improve the bonding ability of the bond to the diamond. The coating acts as a bonding bridge between the two, firmly bonding the diamond and the bond, and improving the bonding strength between the abrasive and the bond.
- Improve the particle strength of the abrasive, and the coating plays a reinforcing and toughening role. The surface defects of diamonds, such as microcracks and small cavities, can be remedied by filling carbide film, thus improving the strength.
- Isolation protection. During high-temperature sintering and grinding, the coating can isolate and protect the diamond from graphitization, oxidation and other chemical reactions.
Dymend introduces 4 commonly used diamond surface coating technologies and their application fields.
Ⅰ Diamond Electroless Plating & Electroplating Nickel and Copper
The extensive application of diamonds and their tools began after the large-scale production of diamonds at high temperatures and high pressure. Diamond can be used to manufacture various cutting and grinding tools, such as saw blades, grinding wheels, etc.
- High-grade diamonds are mainly used to make tools such as saw blades and drill bits.
- Low-grade diamonds are often used to make resin-bonded grinding wheels. In the manufacturing process of resin-bonded grinding wheel, RVD or JR1 type diamond is used. The diamond of this grade generally has the shape of a sheet or thin strip, and the surface is relatively rough, which is conducive to the effective control of the resin matrix on the diamond abrasive particles.
But even so, the efficiency and service life of diamond tools are low, and the manufacturing and use costs are high because the abrasive grains are easy to fall off and break, and the effectiveness of the diamond cannot be fully exerted. This problem has been puzzling to diamond tool manufacturers and tool users for a long time.
In order to improve the service life of resin-bonded diamond wheels, electroless plating & electroplating of Ni, Cu and other metals are used on the surface of diamond particles.
Electroless plating is to deposit metal on the diamond surface through the oxidation-reduction reaction of the autocatalytic process under the condition of no current, typically including diamond plating of Ni, Co, Cu and other metals, or composite plating of Ni-W, Co-W alloys. Diamond is an insulator and cannot be electroplated, but after chemical plating, its surface is metallic and can continue to be electroplated to obtain the required coating type and thickness.
The chemical plating and electroplating products of diamonds are generally used for resin-bonded abrasive tools, which can increase the surface roughness of particles and improve the holding power of resin on abrasive particles. At the same time, the metal after plating can make up the internal defects of particles, improve particle strength and slow down thermal shock.
However, it should be noted that electroless plating or electroplating of Ni, Co and Cu on the diamond surface is not suitable for metal-bonded tools. Even products plated with Ni-W and Co-W alloys cannot be used for metal-bonded tools, because Ni and Co are iron group elements and cannot play a metallurgical role.
This is clearly stated in foreign diamond brands, such as the CDA55N (nickel plating) and CDA50C (copper plating) of De Beers, and the RVG56 and RVG30 of GE in their product descriptions. Nickel-plated diamonds have been misused for making metal bond tools for a long time in China, and this phenomenon is now being corrected.
Ⅱ Diamond Coating with Spiny Corundum
After the diamond is coated with tough metals such as Cu and Ni, the service life of resin-bonded tools can be significantly improved and the use cost of tools can be reduced, but the sharpness and processing efficiency of tools are also greatly reduced. In order to solve the problem of reducing the self-sharpness of diamond particles after coating Cu, Ni and other metals, Wang Yanhui and others developed a new product of “diamond coated with corundum” and related process equipment through years of research and exploration. Diamond coated with corundum can not only improve the holding power of resin matrix to diamond particles, but also keep the original self-sharpening of diamond, solve the contradiction between the service life and use efficiency of diamond tools, comprehensively improve the performance of diamond tools, reduce the manufacturing and use costs of diamond tools, and improve economic benefits.
The technical key points:
Using low melting glass as the binder, the corundum fine particles are sintered on the surface of the diamond particles, so that the diamond surface forms a very rough and good self-sharpening coating, so as to achieve the purpose of not only improving the holding force of the resin matrix on the diamond abrasive particles, but also maintaining the good self-sharpening of the abrasive particles, and greatly improving the service life and working efficiency of the diamond resin abrasive tools.
The main process method:
By using a temporary binder, the glass powder, corundum micro powder and diamond particles are uniformly granulated with a rolling granulator to obtain a granular substance with a mixture of glass powder and corundum micro powder on the surface of each diamond particle, and then the granular substance is dispersed in a large number of corundum micro powder for burying and burning, and corundum coated diamond products can be obtained through screening separation and particle shaping. The control of its weight increase mainly depends on the control of granulation size in the granulator.
Compared with uncoated diamonds, the geometrical appearance of corundum-coated diamonds has changed greatly. The surface of an uncoated diamond is smooth, the surface of corundum coated diamond is very rough, and the shape of the original diamond can hardly be seen. The near needle-shaped corundum particles adhere to the surface of the diamond, making the diamond appear as a spiky ball, as shown in the following picture. Low-melting glass, a material with good brittleness in itself, is selected as the binder. Through its bonding effect, corundum micro powder particles are firmly coated on the surface of diamond particles, making the diamond have uneven thorny surface morphology, greatly improving the holding ability of resin matrix to diamond while maintaining the self-sharpness of diamond, extending the service life of diamond resin tools, and improving the work efficiency.

Ⅲ Vacuum Coating Affinity Metals on Diamond
Diamond metal bond tools made by the sintering method are the leading products in diamond products. Due to the high interface energy between the covalently bonded diamond and the metal bonding phase, the bonding ability between diamond abrasive particles and the bonding metal is poor. There is no interface binding force between the diamond and the matrix binder, the diamond is mechanically embedded in the matrix. The exposed abrasive particles very easy to fall off during operation, resulting in short life and low efficiency of diamond tools. Therefore, diamond manufacturers attach great importance to improving the bonding ability of the metal alloy to the diamond, and improving the bonding state between the bonding medium and the diamond.
At present, it is an effective method to solve the problem of interface bonding between diamond and binder to coat the diamond surface with metal and alloy with affinity, such as Ti, Mo, V, W, Cr, etc., so that they can form metal or alloy layer on the surface of diamond particles through chemical bonding. In the sintering process of tool manufacturing, the corresponding compound layer is formed between Ti and other coatings and diamond, and the metal coating and bond are brazed, so as to achieve the metallurgical combination of diamond and bond so that the abrasive particles do not thresh when working, which greatly improves the working efficiency and service life of the diamond tools. These metals can be deposited on the surface of a diamond by vacuum plating.
At present, the commonly used vacuum plating technologies are as follows.
① Vacuum Physical Vapor Deposition
Under vacuum conditions, metal is vaporized into atoms, molecules or ions and directly deposited on the surface of the plating piece, which is called physical vapor deposition (PVD). Different gasification methods of film-forming materials can be divided into vacuum evaporation plating, vacuum sputtering plating and vacuum ion plating.
Zhu Yongfa used magnetron sputtering technology to coat chromium on the surface of diamond particles, and AES was used to analyze the interface reaction and interface products of diamond and lattice coating when annealed at 300~600 ℃. Based on the vacuum PVD method, multi-layer composite coating technology has been developed. First, titanium or chromium plating by vacuum sputtering, and then nickel cobalt or silver plating by the electrochemical method can play a better role in composition transition. Other Chinese researchers also used vacuum evaporation, magnetron sputtering and ion beam plating methods to coat the diamond surface with titanium, chromium titanium alloy, etc., and studied the interface reaction and bonding between the coating and diamond.
However, PVD technology has some shortcomings in the coating of the granular abrasive surfaces. First of all, the amount of single plating is very small. Under vacuum, metal particles moving at high speed from the evaporation source can only be deposited on the deposited diamond surface, and cannot be deposited in depth. The amount of coating can be increased by ultrasonic vibration of the material chassis or rotation of the inclined material tray to turn the abrasive, but it still can not meet the needs of industrial production, and the coating is uneven and easy to leak. Secondly, due to the low plating temperature, there is only physical adhesion between the coating and diamond after plating, and there is no chemical gold treatment combination. Although the coating can react with the diamond to form a chemical bond during short-time hot pressing, it is obviously not enough to realize the strong bond between the coating and the diamond. The post-plating heat treatment can be used to improve the coating structure. In order to obtain the best coating structure, the process of repeated plating and heat treatment is required, which is difficult to achieve in industrial applications.
② Vacuum Chemical Vapor Deposition
Chemical vapor deposition (CVD) is a coating formed by the chemical reaction of gaseous substances on a solid surface under certain pressure, temperature and time conditions. CVD generally introduces the gaseous compound of the metal to be plated into the reaction chamber where the plated part is placed and forms the coating by thermal decomposition or chemical reaction when contacting the workpiece.
Soviet researchers have extensively studied the CVD method for coating active metals on diamond or cBN, including Cr, Ti, Mo and their alloys, and Zarnoch has also carried out CVD coating on diamond films. In these CVD coating methods, the coating reacts with the diamond chemically to form the corresponding carbide bond, but the reaction temperature is generally 900~1200 ℃, which damages the diamond. At the same time, like PVD, there are problems such as the reaction gas phase’s difficulty penetrating the accumulated internal particles, the single plating amount being low, and the plating cost being high.
③ Vacuum Micro Evaporation Plating
In order to solve the problems of diamond coating by PVD and CVD, Wang Yanhui mixed the diamond with titanium trichloride and titanium hydride as the reducing agent. During vacuum heating, titanium trichloride volatilized and reacted with H2 separated from titanium hydride to reduce metal Ti atoms. These gaseous active Ti atoms are deposited on the diamond surface to form a coating. This method is called “vacuum micro evaporation coating” technology.
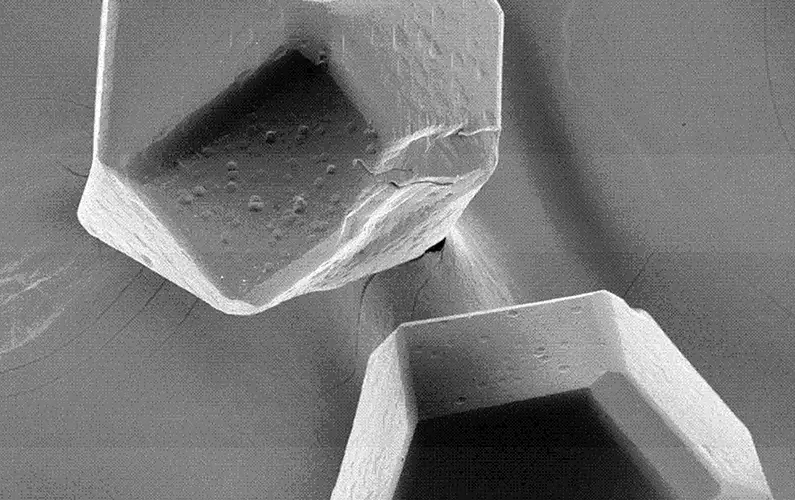
The main technical principle of vacuum micro evaporation plating is to form a uniform vapor environment around each particle, which fundamentally solves the problem that traditional PVD and CVD methods can not deposit stacked granular materials in a single large batch due to the single material emission source. The coating is uniform without leakage. The process parameters shall be selected at the temperature at which the compound can be generated without thermal damage to the diamond so that the compound can be generated during the coating process, control the metal deposition rate, promote its expansion along the interface, and form a completely continuous film covering the diamond surface. The structure of the titanium-plated diamond is shown below.
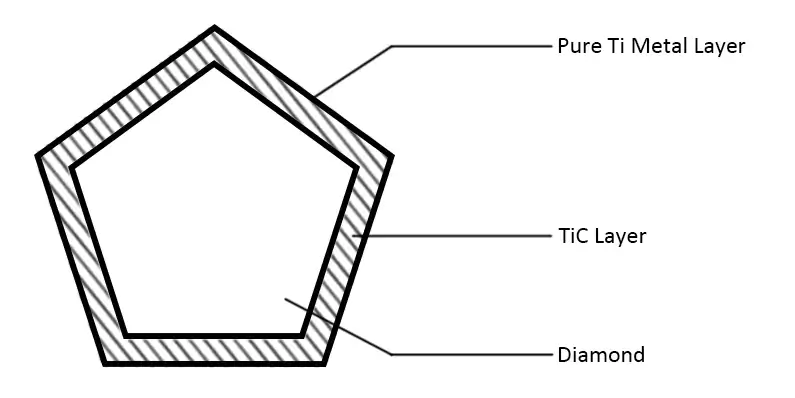
The vacuum micro evaporation Ti-coated diamond has a good interface structure and excellent performance, which provides key common technology for the manufacturing of superhard composite materials. In recent years, diamond vacuum micro evaporation coating technology has been widely used in industry, which has played an important role in improving product quality, reducing production costs and developing new products. Its main functions are as follows:
- The diamond coated with titanium or titanium alloy realizes the strong welding between the diamond particles and the matrix (matrix). The diamond does not fall off and lost during the working process of the tool, and the exposed height (blade height) of the diamond particles on the working face increases, which is the main reason for titanium coating to improve the service life and working efficiency of the tool. The increase in the number of diamond particles on the tool cutting surface after titanium plating (due to no threshing) is equivalent to the increase in diamond concentration, and the increase in the cutting edge height is equivalent to the coarsening of diamond particles. Therefore, the use of titanium-coated diamonds can not only reduce the number of diamonds but also use more fine-grained diamonds. On the premise of ensuring the high performance of the tool, the manufacturing cost of the tool is greatly reduced by using titanium-plated diamonds.
- It is well known that the strength of the diamond in the bit will be significantly reduced after the diamond is sintered into the bit. This is because, during the sintering process of the bit, the graphitized elements such as iron, cobalt and nickel in the formula will graphitize the diamond surface. It can be seen from the broken bit fracture that the diamond is black, so the strength and impact resistance of the diamond will be reduced. The surface of the micro evaporation titanium plated diamond is a continuous titanium carbide film and titanium alloy surface strongly bonded with the diamond. The high melting point titanium carbide layer separates the contact between the diamond and iron, cobalt and nickel, and protects the diamond from thermal damage during the sintering process of the cutter head. Ti-coated diamond was separated from sintered cutter head by corrosion, and its strength did not decrease. In this case, the grade of the diamond used can be appropriately reduced, and the manufacturing cost can be further reduced on the premise of maintaining the high performance of the tool.
- A large number of industrial application results show that titanium plating or titanium alloy is suitable for various strengths and crystalline diamonds. Type I and II diamonds are titanium plated to manufacture metal-bonded grinding heads, grinding blocks, grinding wheels, honing bars, etc. Type III and above diamonds are used to manufacture various saw blades, grinding rollers, and rollers. Without exception, diamond plating greatly improves tool life and efficiency, and reduces tool manufacturing costs. It should be pointed out that inappropriate use of too low strength and low concentration of diamond in tools may result in inadequate plating effect. This is because of too low strength and low concentration of diamond tools, most of the diamond is broken during cutting and grinding, resulting in the failure to fully show the effect of titanium plating.
- For the diamond coated with titanium by vacuum micro evaporation coating technology, through the systematic optimization of coating parameters, a strong metallurgical combination is formed between the diamond and the coating after coating (the coated diamond is rubbed with sandpaper or ground with a ball mill, even if the diamond is broken, the coating will not fall off). When the tool is sintered, the bond and the coating are welded together, so that the diamond and the matrix are strongly welded together. In order to avoid the easy oxidation of pure titanium coating under high-temperature sintering conditions, pure metal titanium may form brittle compounds with some matrix components, which may affect the use effect. Since 1995, the surface layer of titanium-coated diamond coated by the coating technology is an anti-oxidation titanium alloy. When the tool head is sintered, the coating does not oxidize and does not form brittle compounds, which is applicable to various matrix formulations. The diamond coated with titanium alloy can be stored for a long time without oxidation and affecting the use effect.
- After titanium plating, the dependence (sensitivity) of diamond tool performance on bond performance decreases. When titanium is not plated, the bond must play two roles. First, it forms a sintered body with the diamond to form a whole tool with a certain strength and wears resistance. Second, the bond must be embedded in the diamond machine to prevent falling off. Therefore, the bond is blunt if it is hard, and short if it is soft. It is extremely difficult to adjust and stabilize the quality. After using the titanium-plated diamond, the diamond and the bond are strongly welded together, and the corrosion loss of Fe, Co, and Ni to the diamond during high-temperature sintering is avoided. As long as the binder is well sintered, the quality is stable, and a large number of cheap raw materials can be used, which are composed of copper alloy, reduced iron powder and the appropriate amount of tungsten carbide. The iron matrix of 5.5~10usd per kilogram can be used for all kinds of saw blades and all kinds of stones. Its quality, performance and price ratio are at the leading level in the industry.
Ⅳ Silicon Plating on Nanodiamond Surface
Silicon plating on the surface of nanodiamonds can effectively improve the oxidation resistance of nanodiamonds and promote the development of its related diamond tools. Dymend analyzed the TG-DSC properties of the uncoated and silicon-plated nanodiamonds and found that the uncoated nanodiamonds showed an obvious exothermic peak when the temperature rose to 496.5 ℃, accompanied by a decrease in mass. This temperature is the initial oxidation temperature of nanodiamond. When the temperature continues to rise to 647.8 ℃, the diamond is almost completely oxidized to carbon dioxide, and the remaining mass is only 1.27%. It shows that the thermal stability of nanodiamond in the air is very poor, and it was completely burned before 650 ℃.
The exothermic peak of diamond oxidation weightlessness still appears at 737.3 ℃ for nanodiamonds after silicon plating, but the weight loss of the sample corresponding to this peak is very small, only 1.5% and the temperature corresponding to this oxidation peak is significantly higher than that of the uncoated nanodiamond. The oxidation of silicon is completed by the diffusion of oxidizing substances from the oxide film to the silicon interior. When the thickness of the oxide layer is less than a certain critical value, there will be an initial rapid oxidation stage, which corresponds to an obvious exothermic peak at 934.6 ℃ in the differential thermal curve. However, once the thickness exceeds this critical value, the growth rate of the oxide film thickness will slow down, effectively preventing the further diffusion of oxygen and protecting the nanodiamond from oxidation. When the temperature reaches 1075.7 ℃, a very obvious exothermic peak appears, which is the interface reaction between diamond and silicon coating to generate SiC.
The morphology of silicon and titanium plating on the surface of nanodiamonds is shown in the pictures below. From the pictures, it can be seen that the coating completely covers the surface of the nanodiamond and has good homogeneity with the diamond. The dispersion of nanodiamonds is obviously improved after coating. In particular, titanium-coated nanodiamonds can be stably suspended in ethanol for more than a month, which solves the problem of agglomeration of nanodiamonds. In addition, this coating method has a common guiding role in the surface coating modification of powders and can be used to coat nanopowders of various shapes and sizes.

Quasi-atomic layer deposition technology can be used for silicon plating on diamond abrasive surfaces. The graphitized elements such as Fe, Co, and Ni contained in the iron-based binder will seriously erode the diamond during the tool sintering process, resulting in a sharp drop in diamond strength. In addition, diamonds are thermodynamically unstable crystals under normal pressure and are prone to transition to graphitized structures at high temperatures. In the hot pressing sintering process of diamond and metal powder, diamonds will also be affected by atmospheric oxidation, which will reduce the service life of products.
Silicon plating on the surface of the diamond can effectively protect the diamond from the corrosion of the atmosphere and bond, and ensure the working efficiency and service life of diamond tools. The bending strength of the sintered metal bond silicon-plated diamond sintered at 1000 ℃ by hot pressing is 16.2% higher than that of the sintered diamond without silicon plating. An unplated diamond is obviously corroded by a bond. The surface of silicon-plated diamond particles is smooth and flat, and there is no etch pit left by the bond eroding the diamond. The junction between the diamond and the bond has a smooth transition and a good connection. Silicon plating on the surface of a diamond with a quasi-atomic layer effectively prevents the graphite elements in the binder from eroding the diamond, and the bond between the diamond and the binder is good. This coating variety can make the cheap iron-based bond diamond tools more widely used.
Conclusion
The surface theory is a very important basic science in modern natural science, and it is a new branch. The achievements of diamond electroless plating, diamond plating spined corundum, diamond vacuum plating affinity metal, and diamond quasi atomic layer deposition researched by development engineers are all important contents in this field.
The diamond surface itself should have a lot of free bonds, but it is usually passivated. As a result, the diamond will fall off when making products, which is just a mechanical encasement. The industry’s R&D personnel have used a variety of methods to make up for its shortcomings and achieved significant results.
When people master these technologies, they must use them scientifically. There is never a universal method. For example, when chemical plating is used for metal bonding products, ideal results will not be achieved. However, it is very desirable to use diamond vacuum plating to coat affinity metals. Therefore, only by adopting the corresponding technology according to the actual situation can the ideal effect be achieved.
Nanotechnology is a very popular technology at present, and diamond nanomaterials can make things better. However, due to the unfavorable factors of too fine diamond, such as easy agglomeration, easy combustion and easy adsorption, further research is needed.
Dymend Tools will continue to explore the development of diamond tools!