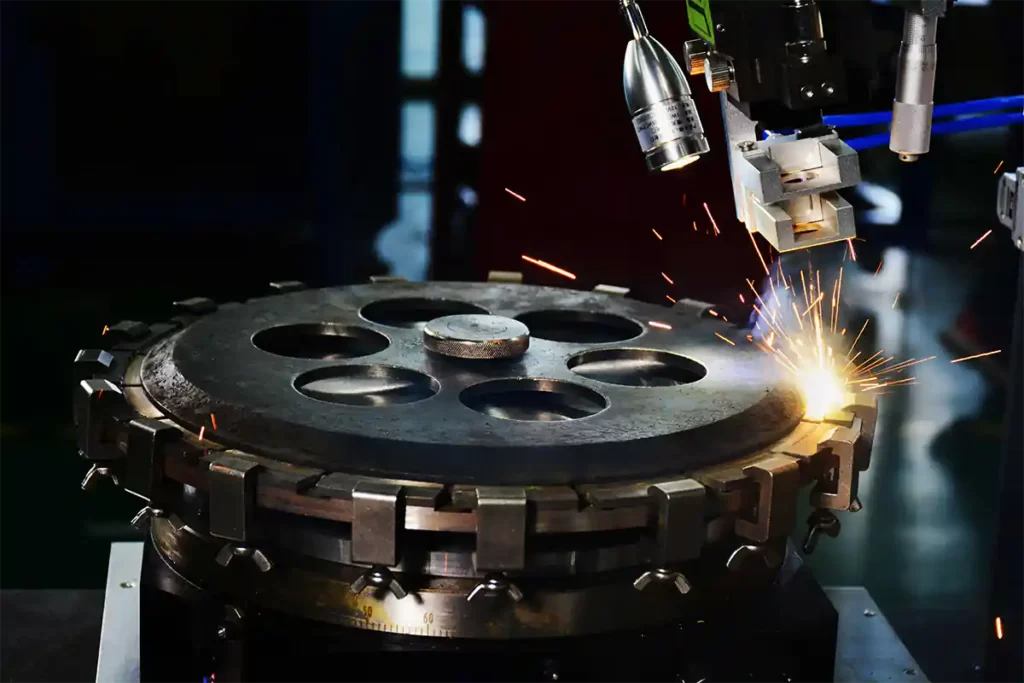
- Preparation Before Electroplating of Diamond Core Drill Bits
- Electroplated Core Drill Bits with Nickel-Iron Alloy Matrix
- Characteristics of Nickel-Iron Alloy
- Basic Formula and Process of Nickel-Iron Alloy Plating Solution
- Effect of Plating Solution on Plating Layer
- Main Salt of Plating Solution
- Sodium Chloride(NaCl)
- Boric Acid(H3BO3)
- Stabilizer
- Influence of Process Conditions
- Brightener
- Softener
- Anode
- Maintenance and Control of Ni-Fe Alloy Electroplating Solution
- Test and Research Methods of Nickel-Iron Alloy Electroplating
- Testing of Electroplated Nickel-Iron Matrix Core Drill Bit
- Using Asymmetric AC-DC Electroplated Iron Base Core Drill Bits at Normal Temperature
The diamond electroplating core drill bit usually uses pure Ni, Ni-Mn or a little amount of Co as the anode, and the steel body of the drill is placed on the cathode, and electroplating is carried out after electrification.
Here Dymend Toos introduces a new method of manufacturing diamond electroplated core drill bits by using nickel-iron and iron matrix and proves the feasibility of this new technology with low price and good quality by explaining the manufacturing principle, method and test comparison.
Preparation Before Electroplating of Diamond Core Drill Bits
Steel Body Processing of Core Drill Bits
The steel body of the diamond core drill bit must be processed and quality controlled according to the design drawings, including the dimensions, materials, etc. The preparation of the steel body mainly refers to the mechanical treatment of the steel body, including grinding, polishing and sandblasting treatment, etc. Where there are angles, all must be ground and polished into a rounded transition.
Organic Solvent Degreasing
Organic solvent degreasing is used to remove saponified and unsaponifiable oils by dissolving them in organic solvents.
Organic solvent degreasing is often used for pre-greasing of parts with serious oil stains. Commonly used organic solvents are acetone, gasoline, kerosene, etc., these substances are expensive, flammable and toxic, and should be used with caution. Organic solvents to clean with hot water, cold water after degreasing. Measure the oil removal is clean or not, see whether the surface of the part by the water cleaning forms a continuous water layer, the larger the area of the water film formed, the cleaner the oil removal.
The advantage of this oil removal method is to remove oil rate faster, no corrosion of metal parts. The disadvantage is that the oil can not be completely removed, because when attached to the metal parts carrying dissolved grease organic solvent evaporates, and part of the oil will remain on the parts and form a thin film of oil. Therefore, after the organic solvent degreasing must also be chemical degreasing or electrochemical degreasing.
Insulation Treatment
The parts of diamond drill bits that do not need plating should be insulated. There are many ways to insulate, such as coating with insulating paint, painting with acetone dissolved celluloid, wrapping with plastic tape, or turning plastic into an inner jacket to insulate the drill bit inside and outside diameter without plating parts in a tight set, and so on. Which specific insulation method should be used according to the drill part, insulation material or insulation requirements, or the combination of two methods that can be used, will be more reliable.
It is worth noting that the insulating material should be able to withstand the electrolyte leaching for a longer period of time, have good adhesion fastness with the steel body, have good acid and alkali resistance and resistance to 70℃ temperature. When plastic tape insulation is used, it is best to use two layers of wrapping, and when painting insulation paint and celluloid, it should be painted more than twice to ensure reliable insulation.
Electrochemical Degreasing
Since organic solvent degreasing is not possible to completely remove the oil, coupled with the insulation process may be contaminated by oil stains, electrochemical degreasing must be carried out.
The composition of the electrochemical degreasing solution and the specification of the degreasing process are as follows.
- NaOH: 15~20g/L
- Na2CO3: 40~45g/L
- Na3PO4: 12~18g/L
- Na2SiO3: 5~8g/L
- Temperature: 25~35℃
- Time: 2~3min for the cathode, 1~2min for the anode
- Current density: 5A/dm2
After oil removal, it needs to be cleaned once with hot water and cold water. Especially when using plastic tape insulation, its surface is easy to adhere to the alkali solution, need to be carefully cleaned. If you do not use hot water cleaning, only cold water cleaning, because the surface of the plated parts by electrochemical degreasing left alkali solution and oil is saponified, emulsified products, after encountering cold water will be cohesive in the surface of the plated parts, resulting in the surface of the plated parts can not be completely removed, thus affecting the bonding strength of the plating. Therefore, it must be cleaned with hot water not less than 70℃, and then cleaned with cold water afterwards. In addition, the degreasing solution needs to be checked, replenished or replaced regularly.
Weak Corrosion
Weak corrosion is divided into two methods: one is the use of dilute acid solution etching to activate the plated surface, and the other is the use of electrochemical anodic activation method to activate the plated surface. But no matter which method is used, the purpose is the same, even if the plated surface may produce a thin oxide film dissolved, exposing the base metal lattice, thus facilitating the deposition of the coating and improve the bonding strength of the coating.
Acid solution weak corrosion usually uses 5%~10% concentration of dilute hydrochloric acid solution, at room temperature etching 0.5~1.0min, then immediately charged into the plating bath.
Anodic activation is to dissolve the oxide film on the surface of the parts to be plated under electrochemical action, revealing the metal lattice, so that the plating layer is firmly combined with the steel body of the drill. The anodic activation solution is 20%~40% sulfuric acid solution, the activation time is 1.0~2.0min, and the current density is 20~30A/dm2 during activation.
The anode should be cleaned with hot and cold water immediately after activation and put into the plating bath with electricity as soon as possible, and never let the plated surface be exposed to air for too long to prevent re-oxidation from affecting the coating bonding and coating quality.
Processes of Electroplating
The plating process of electroplated diamond core drill bits can be divided into two major parts: pre-plating in the tank and composite plating with diamond.
Pre-plating with Electricity in The Tank
The process of pre-plating into the tank is very important, and there are two issues that should be paid attention to.
Firstly, the anode activation should be connected to the power supply immediately after plating, not after the plated parts enter the plating tank and then connected to the power supply plating. This can ensure that electrochemical deposition occurs as soon as the plated part enters the plating bath, so that the plating layer is firmly bonded to the steel body.
Secondly, at the moment when plating starts in the bath, it is necessary to apply a current 2~3 times higher than the normal current density to the plated part for plating. The high current shock increases the overpotential of hydrogen, which can reduce the degree of hydrogen precipitation and improve the quality of plating, and also can improve the uniform plating ability, so that the surface of the plated parts can be plated with a layer of nickel alloy evenly in a short time. The time of high current impact plating is controlled at 2~3min, and then transferred to normal current density for plating. High current density shock plating is especially necessary for complex shaped parts and for plating in electrolytes with high complexing agent and low concentration of metal ions.
After charging into the tank and high current impact electroplating, it returns to the normal current density. This electroplating process is called pre-plating. Pre-plating generally takes 20~30min. Due to the short impact time of high current, it is impossible to coat a uniform coating on the surface of the drill bit. The purpose of pre-plating is to first coat the surface of the drill with a thin layer of metal or alloy, and then add diamond for composite electroplating. In this way, it can prevent the diamond from being directly added to the surface of the rigid metal of the drill bit, which affects the bonding force between the composite coating and the steel body. Instead, the diamond is added on the coating to improve the bonding force between the composite coating and the steel body.
Composite Plating with Diamond
After pre-plating in the tank, at the same time of electrodeposition, the diamond is evenly added to the surface of the metal to be plated in a certain way. in the deposited metal layer. From adding diamonds to impregnating all diamonds in the composite electroplating metal layer, a diamond composite electroplating cycle is completed. This cycle is repeated several times until the thickness of the composite coating reaches the design height of the working layer of the diamond core drill bit.
The length of the composite electroplating cycle time for a diamond drill bit should be determined according to factors such as diamond particle size, current density, and the temperature of the electroplating solution. Generally speaking, the diamond grit is 50/60#, the current density is 1.5~1.6A/dm2, the current density is 1.5~1.6A/dm2, the temperature of the electroplating solution is in the range of 30~35℃, and a composite electroplating cycle can be completed in 12 hours without stirring. If the current density is increased to 2.4~2.5A/dm2, and other conditions are basically the same, a composite electroplating cycle can be completed in 8h.
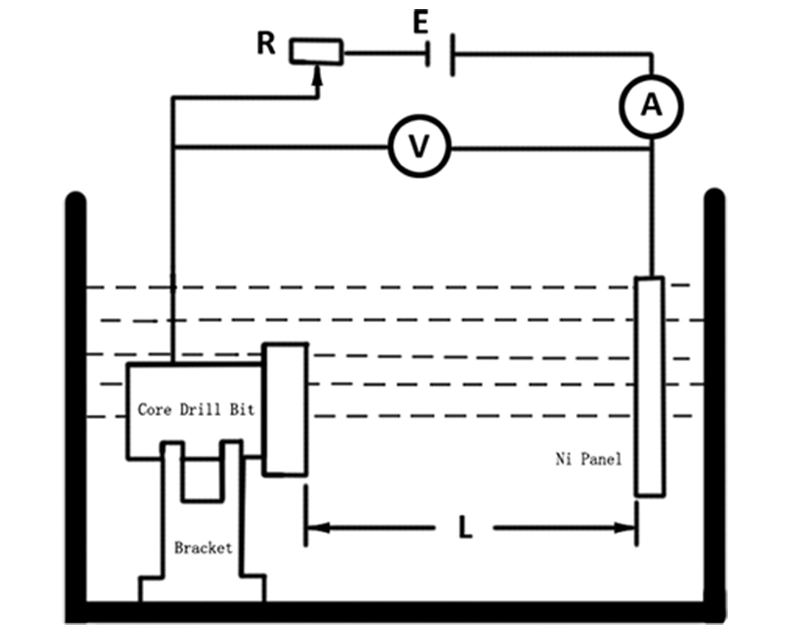
Post-treatment of Electroplated Diamond Core Drill Bits
After the electroplated diamond core drill bit reaches the design requirements, it should come out of the tank, remove the insulation layer, clean and carry out the de-hydrogenation treatment, then trim the surface, for anti-rust treatment and decoration, then the diamond core drill bit is finished. This series of processes is called electroplated core drill bit post-treatment. In the post-treatment processes, hydrogen treatment is the most important.
During the electroplating process, hydrogen precipitation inevitably occurs while the metal is deposited, and part of the precipitated hydrogen stays on the surface of the coating and penetrates into the coating lattice. Hydrogen in the plating layer makes the plating layer and steel body bonding decrease, the brittleness of the plating layer increased significantly, directly affecting the performance and quality of diamond drill bits. Therefore the plating process must minimize hydrogen precipitation, or let the precipitation of hydrogen as soon as possible leave the surface of the coating, as little as possible to penetrate the coating lattice. At the same time after plating, should take measures to let hydrogen escape from the plating, in order to reduce or eliminate hydrogen precipitation to the brittleness of the plated layer and the negative impact on the quality of the drill bit should be reduced or eliminated.
At present, a simple and feasible method of hydrogen removal is to place the plated core drill bit in a constant temperature drying oven for hydrogen removal treatment. The temperature is set at 180~220℃ and the time is set at 2~3h. When the time is up, turn off the power and let the core drill bit cool down with the oven.
Electroplated Core Drill Bits with Nickel-Iron Alloy Matrix
Usually, iron is a harmful impurity in nickel electroplating solution. When the content of iron in nickel electroplating solution exceeds 0.05g/L, it will cause black streaks, darkening of the coating, and foaming of the coating, which will seriously affect the quality of the coating. However, studies have found that nickel and iron are not incompatible in electroplating. Under certain additives and process conditions, a nickel-iron alloy coating with good effect can be obtained.
Characteristics of Nickel-Iron Alloy
- Nickel-iron alloys can be directly electroplated on the iron substrate or used as an intermediate coating. Like electroplating nickel-cobalt alloys, the process is simple and easy to operate.
- It is more convenient to convert ordinary nickel plating solution and bright nickel solution into nickel-iron alloy plating solution. The iron content in the nickel-iron alloy coating can reach 20% to 35%, and replacing nickel with cheap iron can reduce the cost.
- The content of nickel sulfate in the electroplating solution is one-third lower than that of ordinary electroplating nickel or bright nickel plating, which reduces the unit price per liter of the electroplating solution and reduces the initial investment.
- Iron impurities in ordinary nickel plating or bright nickel plating solutions are harmful components, but they become useful components in electroplating nickel-iron solutions. Therefore, nickel-iron plating solutions can eliminate quality failures caused by iron impurities.
- The toughness of the nickel-iron alloy coating is good, which is beneficial to the post-plating deformation processing of tubular parts and other parts.
- When the iron content is higher, the nickel-iron alloy has higher hardness and wear resistance. Therefore, the nickel-iron alloy matrix diamond core drill bit has good adaptability to the drillability of grade 5~9 relatively complete rock layers, it also has higher aging efficiency than the nickel-cobalt alloy matrix core drill bit for drilling hard and dense rocks. At the same time, it is suitable for the manufacture of diamond abrasive tools and saw blades.
Basic Formula and Process of Nickel-Iron Alloy Plating Solution
After experimental research, the basic formula and process of nickel-iron alloy electroplating solution for electroplating diamond bits are as follows. According to different requirements, on the basis of the basic formula, by adjusting the content of each component and adding different additives, different plating solution formulas and corresponding processes for electroplated nickel-iron alloy matrix diamond core drill bits can be obtained.
- NiSO4·7H2O: 180~220g/L
- NaCl (or calculated capacity NiCl2): 20~30g/L
- FeSO4·7H2O: 15~40g/L
- Na3C6H5O7·2H2O: 20~30g/L
- H3BO3: 35~40g/L
- C12H25SO4Na: 0.1~0.3g/L
- C6H5SO2Na·2H2O: 0.1~0.4g/L
- “791” Brightener: 2~6mL/L
- Saccharin: 1~3g/L
- pH Value: 3.2~3.8
- Temperature: 55~65℃
- Current Density: 2.0~3.0A/dm2
- Anode: Ni:Fe=(8~10):1
- Cathode-Anode Ratio: Cathode:Anode=1:2
- Cathode Status: Cathode moving or stirring
Effect of Plating Solution on Plating Layer
Main Salt of Plating Solution
Nickel sulfate is the main salt that provides nickel ions in the plating solution, and its content is generally lower than that of bright nickel plating solution, about 220g/L. Ferrous sulfate is the main salt providing ferrous ion (Fe2+), which contains 20.1% ferrous ion. For each formula, the content of ferrous ion should vary according to the nickel sulfate content, basically in the range of 15~22g/L.
The precipitation of Ni-Fe alloy is anomalous co-deposition, although the electrode potential of Fe ion in the alloy plating solution is 200 mV more negative than that of Ni, Fe still precipitates preferentially on the cathode. Even if the concentration of iron ions in the plating solution is very low, iron will still precipitate preferentially. Thus, it is clear that controlling the concentration of iron ions in the plating solution is the key to obtain nickel-iron alloy plating in practice.
The Trend of Ferrous Ion Oxidation to Fe3+
The characteristics of nickel iron plating solution are that ferrous ions always tend to oxidize into Fe3+ during electroplating, mainly due to the following reasons.
① Natural Oxidation
Whether in the electroplating process or not, the oxidation trend of ferrous ion is inevitable, but the oxidation rate is different due to different conditions. For example, with different stabilizers, under natural conditions and at the same time, the growth value test data of Fe3+in nickel iron plating solution are as follows.
| Types of Stabilizers | Concentration of Fe3+ in the Plating Solution/*10-4g·L-1 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stabilizer H | 2.5 | 3.3 | 4.0 | 5.3 | 5.8 | 6.5 | 7.6 | 7.8 | 8.1 | 8.5 |
| Stabilizer WD-2 | 1.3 | 2.1 | 3.1 | 3.1 | 3.2 | 3.2 | 3.3 | 3.4 | 3.5 | 3.4 |
It can be seen from the table that stabilizer WD-2 is stronger than stabilizer H in inhibiting Fe3+ growth under natural conditions.
② High pH
When the pH value is higher than 3.8, continuous electroplating for a long time may increase Fe3+, which is an inevitable trend.
③ Nickel Anode Area is Too Small
When the area of the nickel anode is small, the anode current density will increase, which will lead to the passivation of the nickel plate, the generation of oxygen, and the oxidation of Fe2+ to Fe3+.
④ The Temperature of Electroplating Solution is Too High
If the temperature is too high, over 65 ℃, the stabilizer will start to decompose, and Fe2+ will be oxidized to Fe3+.
⑤ Stirring Method
Strong agitation with air is easy to promote the oxidation of Fe2+. Therefore, low pressure and weak stirring should be adopted, and continuous filtration should be used to remove the ferric hydroxide solid particles of ferric iron in time.
⑥ The Effect of The Relative Concentration of Fe2+ and Fe3+
The electroplating solution is prepared with a divalent iron salt, but when the pH value exceeds 3.8 or the anode passivation, vigorous stirring and other factors will cause Fe2+ to be oxidized to Fe3+ when the Fe3+ content exceeds 50% of the total iron in the solution, which will affect the leveling and current efficiency of the plating solution and reduce the toughness of the coating. However, under normal circumstances, F3+ will be continuously reduced to Fe2+, so in most cases Fe3+ will not exceed 30% of the total iron content.
Effect of Ferrous Ion Content on Iron Content in Alloys
When the concentration of nickel ions in the electroplating solution is kept constant, the concentration of ferrous ions in the electroplating solution is changed, thereby changing the concentration ratio of metal ions. When the pH value of the electroplating solution is 2.5, the temperature is 52℃±1℃, and the Dk=3.5A/dm2, the nickel ion concentration is 67g/L, and the test is carried out. The effect of the iron ion concentration in the electroplating solution on the iron content in the coating is shown in the diagram below. It can be seen from the curve in the figure that changing the Fe2+ concentration, that is, changing the [Ni2+]/[Fe2+] ratio, with the increase of the iron ion concentration in the plating solution, the iron content in the coating increases linearly. When the iron ion concentration in the plating solution is 5g/L, that is, when the ratio of nickel to iron is 67/5=13.4, the iron content in the coating is 20% (mass fraction).
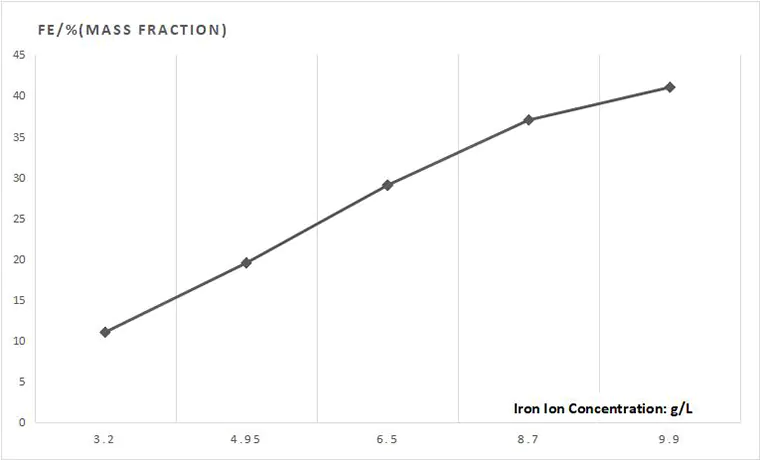
Effect of Nickel-Fe Ion Ratio on Alloy Composition
The influence of the ratio of nickel to iron in the electroplating solution on the iron content in the coating has been tested, and we have concluded the following rules.
| Metal Ion Ratio In Electroplating Solution | 4.0 | 4.7 | 6.3 | 8.0 | 10.6 | 14.0 | 18.8 | 30.0 | 35.0 |
|---|---|---|---|---|---|---|---|---|---|
| Iron Content In Alloy/% | 63 | 54 | 45 | 34 | 32 | 27 | 18 | 12 | 10 |
From the test data, it can be seen that the Ni-Fe ratio in the plating is less than the Ni-Fe ratio in the plating solution, and Fe will be deposited earlier than Ni. In the production, the metal ion ratio in the plating solution is mainly controlled to control the composition of the alloy plating. In the preparation of low-iron, medium-iron and high-iron alloy plating solution, you can refer to this test data to adjust the content of ferrous sulfate without changing the concentration of nickel ions.
Sodium Chloride(NaCl)
The Functions of Chloride Ions(Cl–)
Sodium chloride mainly provides chloride ions for the electroplating solution. Chloride ions can promote anode activation, prevent the passivation of anode nickel plates, and keep nickel ions and iron ions in the plating solution normally dissolved. If the chloride ion content is too high, the nickel ion concentration will increase, and the normal ratio of nickel and iron will be lost, so the concentration must be controlled within the specified range. At the same time, the sodium ions in sodium chloride can increase the conductivity and coverage of the plating solution, reduce the cell voltage and save energy.
Advantages and Disadvantages of Sodium Chloride(NaCl)
Excessive chloride ion concentration in sodium chloride will increase the stress of the alloy coating, so its concentration should be controlled within the specified process range. If the stress is too high, additives such as primary brighteners such as saccharin can be used to reduce the internal stress, so the amount of saccharin is higher than that of bright nickel plating, generally 2~3g/L.
The high concentration of sodium ions in sodium chloride will also increase the stress of the coating, but the influence of sodium ions in nickel-iron solution is not as great as that of bright nickel plating solution. In order to avoid the accumulation of sodium ions due to the long-term supplementation of sodium chloride, nickel chloride can be used when the supplementary chloride ions are insufficient. The amount of chlorine per 2g of nickel chloride is equivalent to the amount of chlorine in 1g of sodium chloride. Or use hydrochloric acid instead of sulfuric acid when adjusting the pH value. The concentrations of hydrochloric acid and sulfuric acid are 12.3 mol/L and 18.2 mol/L, respectively. When the equivalent concentration ratio is 3:1, use 3 mL of concentrated hydrochloric acid (36%) instead of 1 mL of concentrated sulfuric acid (98%) to adjust the pH value, but the chloride ion When the content is high, the pH value cannot be adjusted with hydrochloric acid.
Boric Acid(H3BO3)
The Functions of Boric Acid(H3BO3)
Boric acid is a good buffer. During the electroplating process, hydrogen ions are consumed by discharge, resulting in an increase in the pH value of the solution. At this time, the hydrogen in the boric acid will dissociate to supplement the shortage of hydrogen ions in the solution and stabilize the pH value in the cathode film, preventing the formation of nickel hydroxide and iron hydroxide. Fluctuations in pH value can bring about a series of plating failures, so some people even think that the control and application of boric acid is the key point to obtain a good coating.
Dosage of Boric Acid(H3BO3)
The general dosage of boric acid is 40~45g/L, which is limited by the solubility and should not be too high. When the boric acid content is too high, the solution should be kept at a high temperature during non-working hours so that the boric acid will not crystallize and need to be heated again when it is used next time.
Stabilizer
The Functions of Stabilizer
Stabilizer is an indispensable component in nickel-iron alloy plating solution. The functions of the stabilizer is to prevent ferrous ions from being oxidized into ferric ions and precipitate, which affects the quality of the coating. The stabilizers used are substances that complex with iron. After Fe2+ is complexed, it has the effect of preventing it from being oxidized to Fe3+.
Types of Stabilizer
The types of stabilizers include simple complexing type, complexing reducing type, mixed type and commercial stabilizers. Sodium citrate, sodium gluconate, potassium sodium tartrate, etc. are commonly used. The application of sodium citrate is mainly introduced here.
Action Principle of Sodium Citrate
Sodium citrate is widely used in nickel-iron alloy electroplating. Because of the complexing principle and complexing form of citric acid ion, it is very stable in alloy plating solution. Even if the pH value of the solution is more than 5, it can keep the stability of the plating solution, which has been confirmed in production practice. However, in the electroplating process, especially in the case of anode passivation or too high anode current density and too high temperature, it will also degrade into oxalic acid, forming insoluble ferric oxalate (nickel) with metal ions in the plating solution, causing roughness and burrs of the coating. This degradation is quite slow in the general process range. As long as activated carbon is used for regular treatment, its influence can be eliminated.
Sodium citrate is a complexing agent of ferric iron, which can prevent ferric hydroxide precipitation. With the increase of trisodium citrate content, the iron content in the coating will decrease. Too high sodium citrate content will affect the leveling property of the electroplating solution, so its content should be properly controlled.
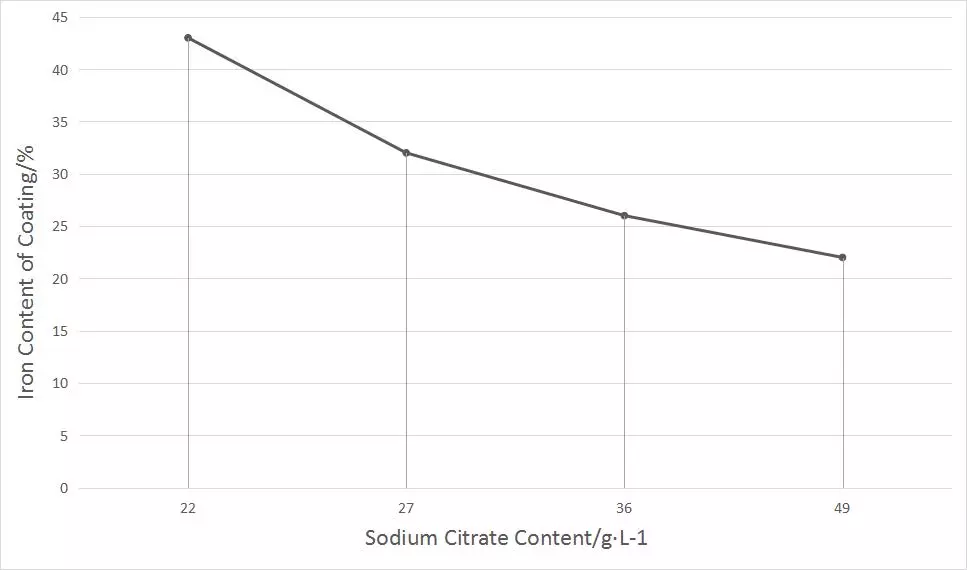
Using of Sodium Citrate
The content of sodium citrate shall be properly adjusted according to the total iron concentration in low-iron, medium iron and high-iron electroplating solutions. When the ferrous sulfate content is 10~40g/L, the sodium citrate content will be kept at 20~30g/L.
When the content of sodium citrate is too high, the toughness and flatness of the coating will be affected, and its degradation will be accelerated.
When the content of sodium citrate is too low, the stability of Fe2+ cannot be maintained, which causes Fe3+ to form hydroxide precipitation and precipitate, resulting in failure.
When sodium citrate is used as a stabilizer, nickel-iron alloy electroplating can only adopt cathode movement, instead of air stirring and barrel plating.
When the concentration of nickel in the solution is constant, the higher the concentration of sodium citrate, the lower the iron content of the coating.
Therefore, when the iron content of the coating is controlled at 25%~30%, sodium citrate should be controlled at 30-40g/L.
Influence of Process Conditions
The Effect of pH Value
The practice shows that the pH value of nickel-iron alloy electroplating solution is controlled within 3.2~3.8, and 3.5 is the best. Within this range, bright, smooth and ductile nickel iron coatings can be obtained during electroplating. However, in the electroplating process, the pH value will gradually increase with the continuous precipitation of hydrogen ions. When the pH value rises to 3.8, the pH value should be adjusted to continue electroplating.
When the pH value is too high (such as more than 3.8), Fe2+is easy to be oxidized to Fe3+, and iron hydroxide will precipitate in the electroplating solution, which will be mixed in the alloy coating, resulting in deterioration of the coating, poor ductility, increased brittleness, decreased smoothness, and burrs.
If the pH value is too low, if it is lower than 3.0, the cathode current efficiency will decrease, which is not conducive to the normal functioning of the brightener, the brightness current density will become narrow, and the leveling performance will decline.
To test the effect of pH value on the iron content in alloy coating, use an electroplating solution with 20% iron content, adjust the pH value to 1.5~4.0 with dilute sulfuric acid, and conduct an electroplating test without changing other process conditions. We will make the following chart for the test results of the effect of the pH value of the plating solution on the iron content in the coating.
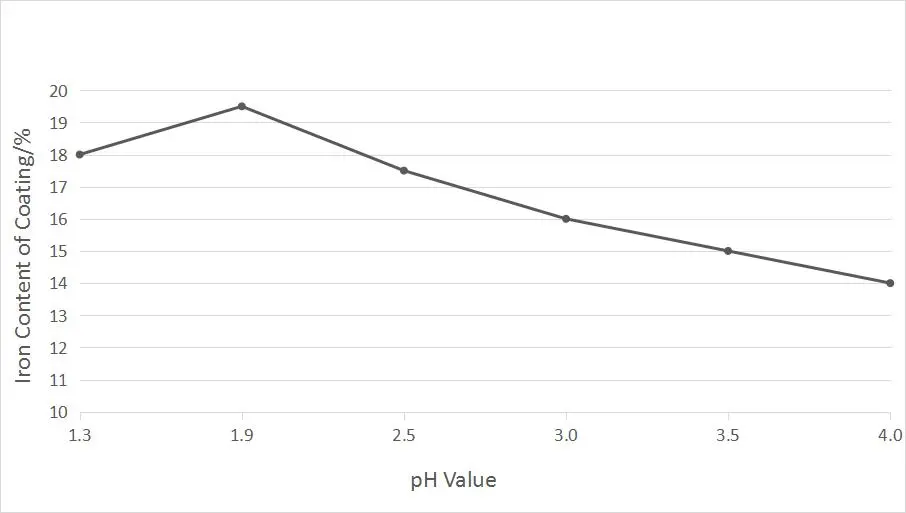
The Effect of Temperature
The experiment and practice show that the optimum temperature of the electroplating solution is 60~65 ℃, and the leveling ability and cathodic current efficiency of the electroplating solution are the best at 63 ℃. The wide range is 55~68 ℃.
If the temperature is too high, it will accelerate the decomposition of the stabilizer and produce harmful degradation products. The continuous high temperature will increase the energy consumption, but it can improve the cathode current density. The anodic dissolution accelerates, the nickel ion content increases, F2+ is easy to be oxidized, and the solution stability becomes poor.
If the temperature is too low, the coating will lose luster, the cathodic current efficiency and flatness will decrease, the upper limit of current density will decrease, the high current density area is easy to scorch, and the coating stress will increase.
The temperature of the electroplating solution has an obvious effect on the iron content in the coating. We conducted the test in the electroplating solution with 20% iron content (mass fraction) in the alloy coating. Under the condition of keeping pH 2.5 and current density 3.5A/dm2, we changed the temperature of the electroplating solution by 30~70 ℃, and drew the influence curve of the temperature of the plating solution on the iron content in the coating as follows.
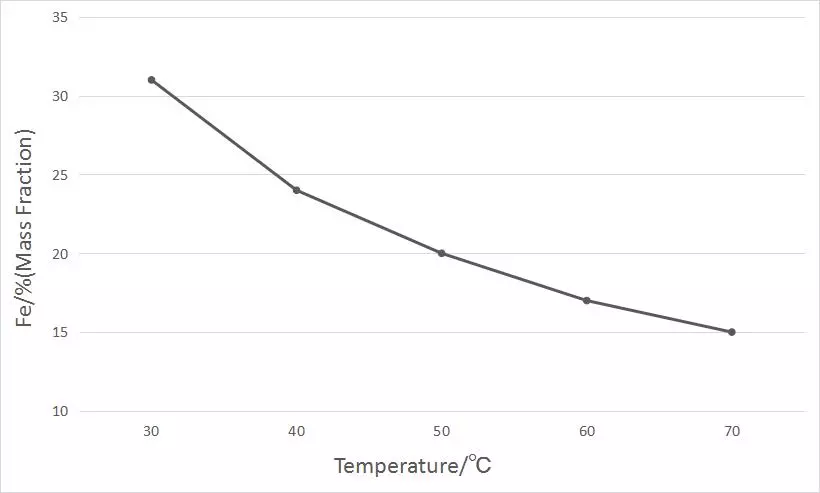
It can be seen from the curve that the increase in temperature causes the iron content in the nickel-iron alloy to change almost linearly, from 31% to 15%, and the iron content is 20% (mass fraction) at about 50 °C.
The Effect of Cathode Current Density
The optimum value of cathodic current density is 3~5A/dm2. Proper high cathodic current density can improve the brightness, flatness and deposition rate of the coating.
If the cathode current density is too high, the coating is easy to be burnt and the pH value of the electroplating solution increases rapidly. The cathode current density is low, the brightness of the coating is insufficient, and the flatness is reduced.
In the electroplating solution containing 20% iron in the alloy coating, we conducted an experiment on the influence of cathodic current density on the iron content in the alloy coating. Under the condition of maintaining pH value of 2.5 and temperature of 52 ℃± 1 ℃, we changed the cathodic current density to 1~10A/dm2. The influence curve of cathodic current density on the iron content in the coating is as follows.
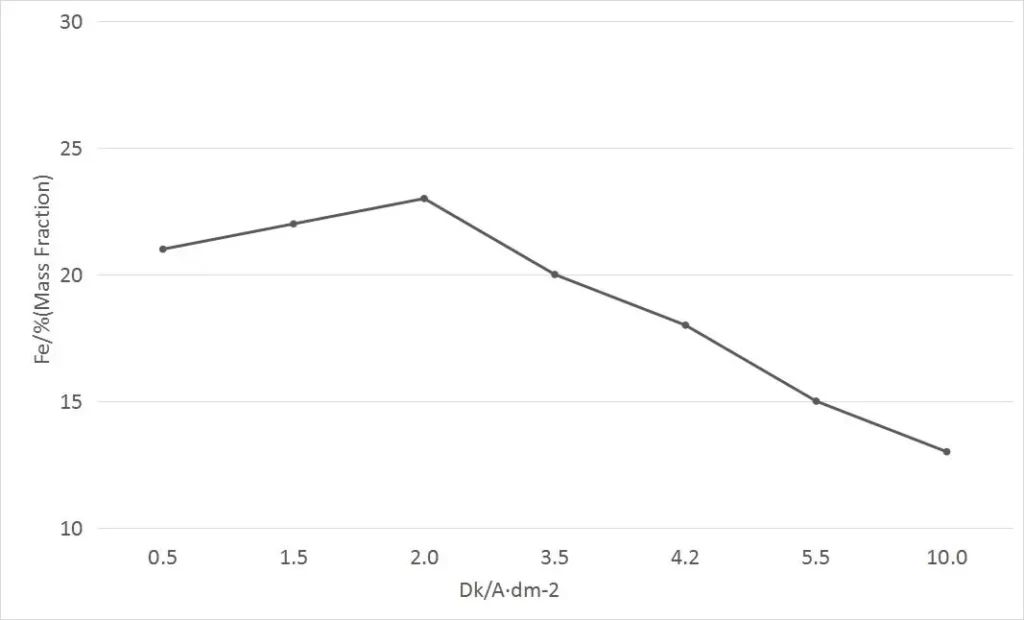
It can be seen from the curve that the current density Dk is within the range of 0-2A/dm2. With the increase of Dk the iron content in the coating gradually increases from 20% to 24%.
When the Dk exceeds 2.0A/dm2, the iron content in the coating decreases gradually with the increase of the current density. When the Dk is in the range of 3~4A/dm2, a nickel-iron alloy coating with an iron content of 20% can be obtained. In the range of Dk of 4~8A/dm2, the iron content is reduced to 15% (mass fraction).
The Effect of Stirring
The influence of stirring on the iron content in the coating varies with the degree of stirring. The intensity of stirring has a great influence on the iron content of the coating. In the same formula or electroplating tank, the iron content of the coating is the highest when air stirring, followed by cathodic movement, and the lowest when no stirring. Therefore, nickel-iron alloy coatings with different iron content can be obtained by using different stirring methods or without stirring.
Strong air agitation will increase the iron content in the coating. For the plating solution with sodium citrate as the stabilizer, it is easy to oxidize Fe2+, resulting in poor stability of the plating solution, so air agitation cannot be used. When the electroplating solution is not stirred, the current density cannot be improved, and the brightness leveling performance is poor.
Brightener
It can be seen from the basic formula that saccharin, “791” brightener and sodium phenyl sulfide are added to the electroplating solution. Saccharin in the electroplating solution is the primary brightener, “791” is the secondary brightener, and sodium benzenesulfonate is also the secondary brightener. “791” is a condensate of 1,4-butanediol, epichlorohydrin and epoxypropane. The main advantages of using it to replace 1,4-butanediol are a wide range of additional amounts, a wide range of allowable current density, good toughness of the coating, fewer decomposition products, and a wide range of flatness and brightness of the plating solution. The additional amount of brightener must be strictly controlled. According to production experience, the appropriate addition amount is:
- Saccharin: 0.02~0.03g/A·h
- “791”: 0.08~0.1mL/A·h
- Sodium Phenylsulfite: 0.005g/A·h
- Sodium Dodecyl Sulfate: 0.0005g/A·h
Sodium dodecyl sulfate is a wetting agent, which has the function of removing pinholes, but it is easy to produce a lot of foam under air agitation. Therefore, when air stirring is used, low foam surfactant is required, instead of sodium dodecyl sulfate as a pinhole remover.
The main reason for burrs in nickel-iron alloy plating is the high concentration of ferric ions in the plating solution. Ferric iron accounts for less than 15% of the total iron, and the working stability of the electrolyte is very high. Therefore, it is necessary to find out the best range of process conditions by testing the process conditions to reduce or eliminate burrs. The basic formula of the nickel-iron plating bath was adopted.
Softener
The brighteners for nickel-iron alloy electroplating are also divided into primary brighteners (i.e. softeners) and secondary brighteners (i.e. main brighteners). Hunan University has conducted comparative tests on several primary brighteners. The results show that saccharin has the best softening and brightening effects, so saccharin is selected as the softener.
In the experimental study, the saccharin content was 1.5g/L, 3g/L and 6g/L respectively, and the brightness of Hull cell test pieces had little difference. However, the saccharin was increased from 1.5g/L to 3.0g/L by the right angle cathode test. The brightness, flatness and bright current density range of the coating are significantly improved. When the saccharin content exceeds 3g/L, various properties do not continue to improve. Therefore, it is appropriate to use 3g/L saccharin content.
However, for the electroplated diamond bit, the saccharin content of 3g/L is more than soft for the coating, but not enough for the hardness and brittleness of the coating. The drilling practice shows that the coating is too flexible, the cutting-edge effect of the diamond is not ideal, and the drilling efficiency is low, which is not the desired result. Therefore, through indoor drilling tests and analysis, it is ideal to control the saccharin content in a nickel-iron plating bath at 1.5~2g/L.
Anode
Nickel iron alloy can be electroplated in a sulfate bath with an alloy anode, nickel-iron alloy sub-control anode, or mixed anode. When an alloy anode is used, it is easy to operate without other auxiliary equipment, but it is not easy to control the concentration ratio of main salt ions in the electroplating solution. The nickel-iron alloy anode (75% nickel, 25% iron) can make the nickel-iron ion content in the solution relatively stable.
In order to control the main salt concentration ratio more accurately, separate anodes or mixed anodes shall be used. When the mixed anode is used, excessive dissolution of iron shall be prevented. Because the solubility of the iron anode is better than that of nickel, the area of the iron anode can only be reduced. When the iron content of the coating is required to be 20%~30%, the area ratio of the nickel anode to the iron anode is (7~8): 1.
In order to obtain a satisfactory coating, the anode should be made of high-purity iron (DT-3), which should be separately packed in a titanium basket and wrapped with polypropylene fabric to prevent anode sludge from entering the plating solution. Pay attention to the dissolution of the iron anode at any time. The current density of the two anodes does not need to be controlled separately, but the distribution should be uniform to ensure that 8%~12% of the current passes through the iron anode. It is better to use active nickel and keep the ratio of the anode area to the cathode area at 2:1.
Maintenance and Control of Ni-Fe Alloy Electroplating Solution
Control of Main Salt Content
In the nickel-iron alloy plating solution, keeping a certain proportion of nickel sulfate and ferrous sulfate can make the iron content in the coating within the required range. For example, when nickel sulfate is 220g/L and ferrous sulfate is 15g/L, the iron content in the coating is about 20%. If the anode current density is 0.8~1.2A/dm2, the nickel anodic dissolution is normal, and the nickel ion of nickel sulfate is supplemented by anodic dissolution, so that the nickel sulfate content is not lower than the upper limit, and it is unnecessary to provide nickel salt. However, the content of ferrous sulfate changes greatly with the increase or decrease of the iron anode. If the content is too low, ferrous sulfate can be directly added. The increase of ferrous ions only by the dissolution of the iron anode will increase the pH value of the bath too quickly.
The electroplating solution should be analyzed once a week to strictly control the ferrous sulfate content in order to ensure the iron composition in the coating. The pH of ferrous sulfate saturated aqueous solution is 2.9. During replenishment, the pH value shall be acidified to 3.5 with distilled water. After ferrous sulfate is dissolved, it shall be added to the electroplating tank after clarification and filtration.
Control and Treatment of Ferric Ion
Effect of Fe3+
The main ferrous sulfate salt in the nickel-iron alloy plating solution exists as ferrous ions. However, due to the increase in pH value, temperature, air agitation and other factors, some ferrous ions are inevitably oxidized to ferric ions. The higher content of the ferric ion, the lower the cathodic current efficiency and the lower the leveling performance of the electroplating solution. However, when the ferric ion is controlled at 10%~20% of the total iron content, it has little effect. Fe3+ can not exceed 40% of the total iron content, because there is a stabilizer complexing Fe3+. However, due to the limited content of the stabilizer, the content of Fe3+ is too high, and the stabilizer can not be fully complete, which will make the harmful effect of Fe3+ significant.
Treatment Method When Fe3+ Is Too High
① Iron powder reduction method. When the Fe3+ content in the plating solution is too high, first adjust the pH value of the plating solution to about 3.0, and then add the iron powder to the plating solution according to the reaction formula: 2Fe3+ + Fe = 3Fe2+.
The amount of iron powder is 1/2 of the amount of ferric iron. Keep stirring and keep the temperature at 40~50 ℃. The reaction is relatively slow. The color of the solution gradually changes from dark to light. The pH value keeps rising. Pure iron powder reacts with hydrogen ions at the same time to release hydrogen bubbles. After the bubble stops, adjust the pH value to 3.0. Then analyze the plating solution. Excess Fe2+ is precipitated by reducing the iron anode or powering on the large cathode to treat part of the iron. After excessive iron powder reduction, Fe3+ cannot be completely cleaned, as long as the coating quality is not affected.
② Reducing agent method. Add ascorbic acid or isoascorbic acid or sodium pyrosulfite to reduce Fe3+ to Fe2+.
③ Low pH natural reduction method. When the pH value is reduced to 3.0 and properly heated, Fe3+ will decrease from 2.6g/L to 1.5g/L. After several days of operation, Fe3+ will be stabilized at 0.6~1.0g/L.
④ Low pH electrolysis method. When the pH value is reduced to 3.0, Fe3+ will be reduced to Fe2+ from the cathode surface after electrification and electrolysis for a period of time.
Maintenance and Control of Fe3+
As long as the process conditions are strictly maintained and implemented, Fe3+ will not increase under normal conditions. A high pH value accelerates the formation of Fe3+. The test shows that when the pH value is adjusted from 3.4 to 5.5, Fe3+ increases from 0.41g/L to 1.12g/L and the ratio to the total iron content increases from 11% to 36%, which has exceeded the limit of 15%. At this time, the excess Fe3+ needs to be reduced with iron powder. It can be seen that strict control and timely adjustment of the pH value of nickel-iron alloy plating solution are extremely important to prevent the rise of Fe3+. The influence statistics of nickel-iron ion concentration ratio on the iron content of the coating are as follows.
| Nickel-Iron Ion Concentration Ratio In Electroplating Solution | 9:1 | 13:1 | 20:1 | 25:1 | |
|---|---|---|---|---|---|
| Iron Content of Coating | Cathode Movement | 32.0% | 25.0% | 20.0% | 14.0% |
| Air Agitation | 30.0% | 23.0% | 17.0% | 12.0% | |
| Static Electroplating | 21.0% | 17.0% | 10.5% | 7.5% | |
Maintain Nickel-Iron Ion Concentration in Electroplating Solution
In order to keep the iron content in the nickel iron alloy coating at about 25%, the ratio of nickel to iron ions in the plating solution should be controlled. The influence of its ratio on iron content in alloy coating is shown in the table above.
It can be seen from the data in the table that when the cathode moves, the iron content in the coating should be kept at 25%, and the nickel iron ion concentration ratio in the electroplating solution should be kept between (10~15): 1.
It is expected that the content of iron in the electrodeposited nickel iron alloy matrix diamond bit is not less than 30%. Under the condition of cathode moving, it is appropriate to control the ratio of nickel to iron ions in the electroplating solution (9~10): 1. In static electroplating, the nickel iron ion ratio in the electroplating solution should be controlled at (8~9): 1.
Test and Research Methods of Nickel-Iron Alloy Electroplating
The performance methods of electroplated bit matrix include orthogonal regression test design method, uniform formula design method, etc. From the composition analysis of the basic electroplating solution for electrodepositing nickel iron alloy, it can be seen that the content of ferrous sulfate and sodium citrate has a direct impact on the alloy composition in the nickel iron coating, and the impact of ferrous sulfate is more significant in both. Sodium citrate mainly acts as a stabilizer to control the content of ferric ion, which can change in a large range. Therefore, only the single factor test method is used for preliminary experimental research on ferrous sulfate content to find out the influence of ferrous sulfate content change on the hardness of nickel iron coating.
The content of ferrous sulfate in the test is arranged according to the equal difference value, that is, 11 tests are arranged for 0g/L, 2.5g/L, 5g/L, 7.5g/L, 10g/L, 12.5g/L, 15g/L, 17.5g/L, 20g/L, 22.5g/L, 25g/L, 11 samples of nickel iron alloy coating are obtained, and hardness tests are conducted for these 11 samples. The hardness data measured are as follows.
| Ferrous Sulfate(g/L) | Hardness Test Value of Samples | Average Hardness(HRB) | |||
|---|---|---|---|---|---|
| 0.0 | 106.4 | 107.8 | 106.6 | 107.2 | 107.00 |
| 2.5 | 103.0 | 104.8 | 107.3 | 109.2 | 106.40 |
| 5.0 | 109.7 | 110.8 | 108.9 | 109.4 | 109.70 |
| 7.5 | 110.3 | 110.5 | 108.4 | 108.9 | 109.50 |
| 10.0 | 112.4 | 112.1 | 112.2 | 110.0 | 110.70 |
| 12.5 | 110.6 | 109.4 | 108.6 | 112.2 | 110.20 |
| 15.0 | 112.6 | 114.4 | 112.8 | 112.1 | 113.00 |
| 17.5 | 112.9 | 114.9 | 113.1 | 112.7 | 113.40 |
| 20.0 | 113.3 | 115.2 | 114.7 | 111.6 | 113.70 |
| 22.5 | 113.7 | 114.9 | 113.2 | 115.0 | 114.20 |
| 25.0 | 116.4 | 115.2 | 113.8 | 115.4 | 115.20 |
Through data analysis of the hardness value tested, we have obtained the following hardness change curve.
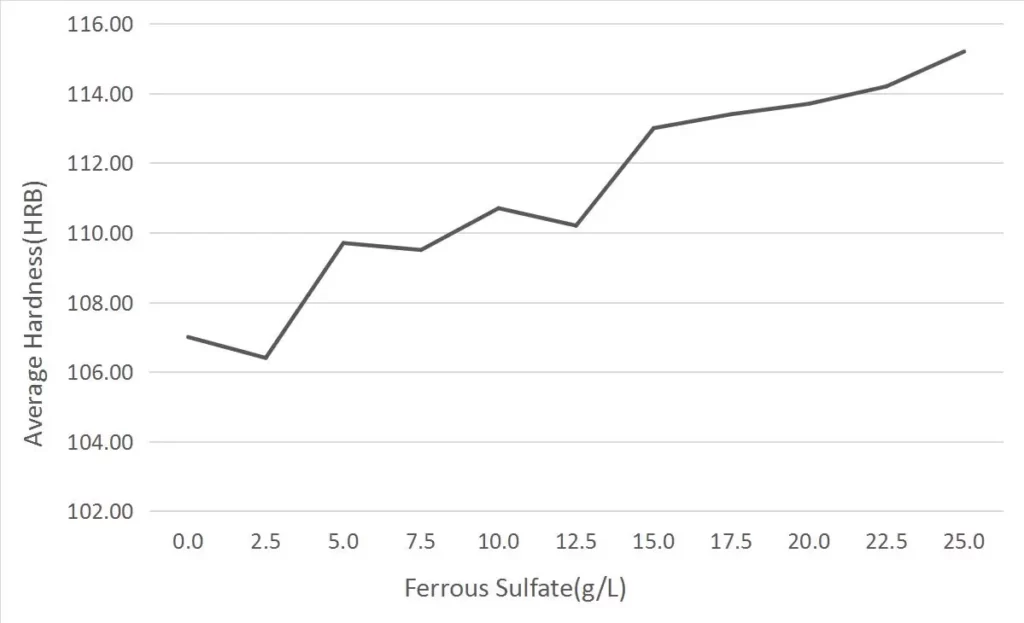
It can be seen from the curve that the hardness of nickel iron alloy coating is increasing with the increase of ferrous sulfate content, and the rule is very obvious. This means that with the increase of ferrous sulfate content in electroplating solution, the iron content in nickel iron alloy will increase, and the hardness of nickel iron alloy will also increase accordingly. At the same time, the hardness test value of the test sample is very consistent with the predicted value after regression analysis, and the change trend of hardness is basically consistent, indicating that nickel iron alloy has a good application prospect as the matrix material of electroplated diamond bits.
It can be seen from the curve that the hardness of the first seven samples changed greatly, from HRB107 to HRB113.0, with an average increase of about 0.9. The hardness of the last four samples changed slightly, with an average increase of only 0.45. Analysis of the causes shows that it should be directly related to the increase of iron ion content in the electroplating solution. The increase of iron ion content and inaccurate temperature control will lead to the increase of Fe3+ concentration in the electroplating solution, and the inclusion of iron hydroxide deposits [Fe (OH)3] in the nickel iron alloy coating, which will lead to the decrease of the compactness and increase of the unevenness of the coating, and ultimately reduce the hardness of the coating. At the same time, the error of hardness test also increases with the coating roughness.
The above test results can be used as the basic basis for designing bit performance for different rocks. By adjusting the content of ferrous sulfate and sodium citrate and the electroplating process, the properties of nickel iron alloy matrix with different properties can be obtained, and diamond bits with different properties can be electroplated.
Electroplated nickel iron alloy matrix diamond bit can replace nickel cobalt alloy matrix diamond bit when drilling in most rock formations;
For hard and dense rock stratum, its drilling effect is better than that of nickel cobalt matrix diamond bit, which not only has higher time efficiency, but also significantly reduces the cost.
Testing of Electroplated Nickel-Iron Matrix Core Drill Bit
The concentration of ferrous sulfate, nickel sulfate and sodium citrate in the electroplating solution of nickel iron alloy matrix diamond bit is 20g/L, 220g/L and 30g/L respectively; The temperature of electroplating solution is 40 ℃, the current density is 1.8A/dm2, and the pH value of electroplating solution is controlled within the range of 3.5~3.6; The diamond concentration is about 95%, and the diamond grain size is mainly 45/50#.

We use three bits for drilling test, and the size of the bit is Φ41/27mm, rock test sample is felsic granite with drillability of grade 9, containing about 55% quartz and medium grain size. During the indoor test, the drilling pressure is 4kN and the drill speed is 400rpm. The test drilling results are shown in the table below.
| Core Drill Bit No. | Number of Boreholes(pcs) | Drilling Depth(m) | Cumulative Time(h) | Efficiency(m/h) | Segment Consumption Height(mm) | Estimated Service Life(m) |
|---|---|---|---|---|---|---|
| No. 1# | 10 | 9.0 | 4.60 | 1.95 | 1.05 | 42.80 |
| No. 2# | 10 | 9.0 | 5.00 | 1.80 | 0.90 | 50.00 |
| No. 3# | 10 | 9.0 | 4.80 | 1.87 | 1.00 | 45.00 |
| Average | 10 | 9.0 | 5.77 | 1.87 | 1.10 | 45.93 |
Note:
- ① The stone sample is a 1m3 cube. Because the stone sample is not very regular, the drilling depth is calculated as 0.9m/piece.
- ② The expected life is calculated based on the working layer with a bit height of 5mm.
It can be seen from the indoor drilling effect that the drilling efficiency reaches 1.87m/h, the wear of the bit is about 0.1mm/m, and the average life of the bit is expected to be 45.93m, which is a relatively satisfactory test and research result. Electroplated Ni-Iron(or Ni-Fe) alloy matrix diamond core drill bit is a new kind of diamond core drill bit, which has been widely used.
Using Asymmetric AC-DC Electroplated Iron Base Core Drill Bits at Normal Temperature
Features and Advantages
Electrolytic iron has high hardness and brittleness due to its hydrogen content. If DC iron plating is carried out at room temperature, in order to obtain a coating with good bonding strength and high hardness, it can only be carried out at low current density. In order to improve the deposition rate of the coating, it is necessary to use a larger current density. At this time, if the temperature of the electrolyte does not increase correspondingly, the amount of hydrogen released from the cathode will increase rapidly, resulting in a large amount of hydrogen absorption by the deposited iron layer. As a result, the grain structure of the coating is seriously dislocated, and the internal stress rises. When it reaches a certain limit, the coating will automatically crack or even become a powder. After the temperature of electrolyte is raised, the reduction rate of hydrogen overpotential is smaller than that of iron precipitation potential. When iron deposition is accelerated, the amount of hydrogen absorption is correspondingly reduced, and the above shortcomings can be overcome to a large extent. However, maintaining the high temperature of electrolyte requires complex temperature rise and control equipment; The rapid evaporation of electrolyte requires frequent adjustment, and the volatilized steam has certain toxicity: the hydrogen content of the coating is still high, the bonding strength with the base metal is still not ideal, etc., which limits the further promotion and application of DC iron plating.
Asymmetric alternating current is used to make the positive half wave of the current greater than the negative half wave. In this way, the iron deposited on the plating piece is always more than the dissolved iron. With the increase of the asymmetry ratio, the deposition rate will be faster and faster.
It is worth pointing out that when asymmetric alternating current electroplating is adopted, the deposition is mainly on the plated parts in each cycle; However, when the plating piece becomes the anode, only a small part of the coating can be dissolved. At this time, the hydrogen contained in the electrode will be preferentially oxidized and removed. Therefore, the hydrogen content in the coating can be greatly reduced by asymmetric AC electroplating.
The reasons for using asymmetric AC can be summarized as follows:
- (1) Reduce the concentration polarization of electrolyte, thereby reducing the hydrogen content of the coating.
- (2) Eliminate the internal stress of the coating and improve the adhesion between the coating and the base metal.
Asymmetrical alternating current is used. When the plating piece is in the negative half wave, its convex peak can be largely dissolved and cut, and more hydrogen permeating parts can also be removed at the same time. The surface of the plating piece is recovered more smoothly, so the structure of the subsequent coating is more consistent. On the other hand, when the current direction changes, the cathodic electric field force no longer plays a role, while the anodic electrochemical force can make the original distorted and dislocated lattice get corrected.
It should be pointed out that the condition of the initial coating is the key factor for the bonding strength. When asymmetric AC electroplating is adopted, start with a very small asymmetry ratio (ratio of forward current to reverse current), usually the asymmetry ratio β It is about 1:1.3, and the cathode electric field force is slightly greater than the anode electric field force, which can ensure that there is enough reverse electric field force to correct the lattice, and most of the hydrogen infiltrated can be eliminated; The internal stress of the initial coating is very small, and the interface change with the base metal is also very small, so the bonding strength is greatly improved. Secondly, for production efficiency, that is, deposition rate and current efficiency, undoubtedly DC plating has a faster deposition rate and higher current efficiency than asymmetric AC plating. Therefore, after the asymmetry ratio is gradually increased, DC electroplating can be applied on the firm initial coating, which is using asymmetric AC-DC electroplated iron base core drill bits at normal temperature.
To sum up, asymmetric AC-DC normal temperature iron plating has a series of advantages over DC iron plating, such as fast deposition rate, high production efficiency, wide source of materials, low cost, easy operation and low toxicity. Therefore, asymmetric AC-DC room/normal temperature iron plating is a promising new process, which can be fully applied to the field of electroplated diamond core drill bits.
Types of Electrolytic Solution of Iron
According to the varieties of the main salts in the electrolyte, three types of electrolytes are mainly used in practice:
- (1) Electrolyte mainly containing ferrous sulfate.
- (2) Electrolyte mainly containing ferrous chloride.
- (3) The electrolyte mainly consists of the mixed solution of ferrous sulfate and ferrous chloride.
There are other types of electrolytes, among which ferrous fluoroborate electrolyte and sulfamate electrolyte are worth mentioning. These different iron plating processes and formulations have their own characteristics.
The properties of iron plating layers obtained from different iron plating processes are quite different, which is greatly related to the pH value, temperature, current efficiency, types and properties of additives of the plating solution. The structure of metal iron obtained by different iron electroplating processes is also different, especially the current density has obvious influence on the structure of metal structure. The metallographic observation of the cross section of the iron plating layer shows that when the cathode current density is 5~15A/dm2, the crystal is columnar. When the current density is 1~2A/dm2, the coating crystallizes into layers. Obviously, with the increase of current density, the hardness of the coating will also increase, which is related to the change of the coating structure.
After the experimental research on the above three types of plating solutions, the low temperature chloride electrolyte was finally determined to be used as the plating solution for electroplating diamond bit, considering the cost of the plating solution, the performance of the coating, the pollution degree of the plating solution and the ease of operation.
Process Formula of Low Temperature Chloride Plating Solution
There are two types of chloride iron plating: low temperature type and high temperature type. High temperature ferrous chloride plating adopts high concentration and high current density, with fast deposition rate, high purity, low hardness, good toughness and low internal stress. However, the stability of the electroplating solution is poor, mainly because divalent iron is easy to be oxidized to trivalent iron, which makes the electroplating solution maladjusted and the coating quality worse.
Low temperature ferrous chloride electroplating solution can save energy, and has the characteristics of fast deposition rate, high current efficiency and high hardness of the coating. As the bath temperature is low, the oxidation rate of ferrous ion is slower, which is conducive to the stability of the bath. Therefore, the application of low temperature iron plating is more than that of high temperature iron plating. At the same time, the low temperature chloride electroplating solution iron plating process should be different from the high temperature chloride iron plating process.
The following is the formula and process of low temperature ferrous chloride plating solution for electroplated diamond core drill bits according Dymend‘s study:
- Ferrous Chloride: 350~400g/L
- Ammonium Chloride: 20~30g/L
- Manganese Dichloride: 8~10g/L
- Boric Acid: 8~10g/L
- pH Value: 2.0~3.0
- Plating Solution Temperature: 35~45 ℃
- Current Density: 3~5A/dm2
The Effect of Process Parameters
Main Salt
Ferrous chloride is the main salt of the bath, and its concentration can vary in a large range. With the increase of main salt concentration, the allowable current density also increases, and the deposition rate can be accelerated, but the hardness of the coating decreases, and the coating is prone to rough crystallization. However, the decrease in the main salt concentration will not only reduce the deposition rate but also increase the hardness and brittleness. The electroplated iron-based diamond core drill bit is different from the repair electroplated iron, so it does not need such a high current density. Therefore, the main salt concentration of the plating solution can be appropriately reduced to ensure the hardness and brittleness of the coating.
Ferrous ions are easy to be oxidized into ferric iron, and the increase in temperature and pH value will intensify the oxidation. Therefore, low pH value and low temperature are beneficial to the stability of the electroplating solution.
Auxiliary Salt
In chloride iron plating, some auxiliary salts are often added to improve the conductivity of the electroplating solution or play other roles. For example, manganese dichloride has the function of refining crystallization, and it can also inhibit the oxidation of ferrous ions, which has a good antioxidant effect. Ammonium chloride can improve the hardness of the coating and slow down the oxidation rate of ferrous ions. At the same time, when the pH value of the solution rises and the concentration of OH– is high enough to possibly precipitate Fe (OH)3, OH– ions will be consumed according to the reaction formula: NH4+ + OH– = NH3 + H2O . It can not generate Fe(OH)3, and NH4Cl acts as a buffer.
pH Value
Since the anode current efficiency of iron plating reaches 100%, while the cathode current efficiency is lower than 100%, that is, the hydrogen ion in the electroplating solution will decrease with the prolongation of electroplating time, and the pH of the plating solution will increase. Therefore, the pH value shall be tested and adjusted frequently during iron plating, and adjusted with hydrochloric acid to control the pH value within 3.0. The increase in pH value will accelerate the oxidation of ferrous ions and produce ferric hydroxide precipitation, and the colloidal substance generated by ferric iron will lead to an increase in coating brittleness and a decrease in coating quality.
Temperature
The increase in temperature can increase the working current density, which can improve the electrodeposition rate. At the same time, with the increase in temperature, the hardness and brittleness of the coating will also decrease, but the stability of the plating solution will also decrease. Therefore, the iron-plated diamond core drill bit does not want to use high-temperature parameters but chooses a low-temperature electroplating process at about 40 ℃.
Cathode Current Density
In the process range, the hardness of the coating increases with the increase of current density. However, after the hardness reaches a certain value, increasing the current density will not increase the hardness, but will make the coating become rough. The electroplated diamond core drill bit itself is added with diamond solid particles. When the coating becomes rough, it is not conducive to the solid embedding of the diamond, and the wear resistance of the bit will decline. Therefore, the current density adopted by the iron-plated diamond bit is relatively low, generally 3~5A/dm2.
Anode
The anode for iron plating shall be pure iron or low-carbon steel with a carbon content of no more than 0.1%. Since the current efficiency of the anode for iron plating is close to 100%, the area of the anode shall be smaller than that of the cathode, which is contrary to the requirements of most other anodes for electroplating. The area ratio of the cathode to the anode can be 1: (0.5~0.7), and an anode bag should be added. When the electroplating solution is not working, the anode should be taken out of the electroplating tank.
Electroplating Process Specification
As the internal stress of low-temperature DC iron plating is large (tensile stress can reach 2000kg/cm), if direct DC plating is carried out without certain pretreatment, the coating will peel or crack. In order to avoid this situation, the method of subsection electroplating is adopted in low-temperature iron plating. The process of using asymmetric AC-DC electroplating iron matrix diamond core drill bit is the same as that of the above nickel cobalt matrix diamond bit (omitted), and the pretreatment process is also very important. The difference is that before DC electroplating, it must go through two important links: starting electroplating and transition electroplating.
Starting Electroplating
Asymmetric AC-DC power supply is used for starting electroplating. The current density when starting plating should be determined according to the basic process of electroplated diamond core drill bit. The cathode current density is 4-5A/dm2 at the positive half cycle, 2-3A/dm2 at the negative half cycle, and the effective current density is 1-2A/dm2. Electroplate for 5~10min to generate a layer of iron coating with low stress and high bonding strength with the matrix of segment.
In the process of industrial iron plating, an etching process is carried out after the plating piece is put into the electroplating tank and before the plating. The purpose is to remove the oxide film that may be passivated on the surface of the plating piece, so that the activated base metal of the plating piece directly contacts the electrolyte. Due to the high acidity of the electroplating solution, α- Fe2O3 passivation film is basically alkaline oxide film, which will neutralize each other. Removing the passivation film can effectively improve the bonding strength between the coating and the substrate. Through practice, Dymend can still obtain high strength coatings by controlling the temperature of the electroplating solution, the current density into the electroplating tank, and the starting and transition electroplating processes.
Here, Dymend uses one electroplating tank to plate 5 core drill bits with Φ130mm at a time as an example.
(1) Calculate the plated area of the drill bit:
The plated area of one drill bit S1=0.70dm2, and the plated area of five drill bits S=S1×5=3.5dm2.
(2) Determine current density:
Forward current density D1=5A/dm2, reverse current density D2=3.8A/dm2.
Effective current density Dk=1.2A/dm2.
(3) Calculate current value and asymmetry ratio β:
Plating forward current I1=D1 · S=5A/dm2×3.5dm2=17.5A.
Plating reverse current I2=D2 · S=3.8A/dm2×3.5dm2=13.3A.
Asymmetry ratio β= I1/I2=17.5A/13.3A=1.32.
(4) Starting electroplating:
After five core drill bits are put into the electroplating tank, the I1 and I2 values remain unchanged, and the plating starts for 10min. After 10min, it can enter the transitional electroplating stage.
Transition Electroplating
After 10 min of plating, fix the forward current I1 unchanged, and gradually reduce the reverse current I2 to 1/8~1/10 of the forward current within 10~15 min, i.e β= 8~10, transfer to DC electroplating after 5 minutes of electroplating. The current adjustment of the transition electroplating is shown in the following table.
| Reverse Current(I2/A) | Forward Current(I1/A) | Asymmetry Ratio(β) | Required Time(min) | Effective Current Density(A/dm2) |
|---|---|---|---|---|
| 13.30 | 17.50 | 1.32 | 0 | 1.20 |
| 10.90 | 17.50 | 1.60 | 1.5 | 1.88 |
| 6.65 | 17.50 | 2.00 | 1.5 | 3.10 |
| 4.43 | 17.50 | 3.00 | 1.5 | 3.73 |
| 3.33 | 17.50 | 4.00 | 1.5 | 4.05 |
| 2.66 | 17.50 | 5.00 | 1.5 | 4.24 |
| 2.22 | 17.50 | 6.00 | 1.0 | 4.36 |
| 1.90 | 17.50 | 7.00 | 1.0 | 4.46 |
| 1.66 | 17.50 | 8.00 | 1.0 | 4.53 |
| 1.48 | 17.50 | 9.00 | 1.0 | 4.58 |
| 1.33 | 17.50 | 10.00 | 1.0 | 4.62 |
Then, gradually reduce the forward current value from 17.5A to 8.33A within 2min. The calculation method of this value is: first, determine that the current density of DC electroplating is 2.0A/dm2, the total area of the five drill bits is 3.5dm2, and the total current is 7.0A, which is the effective current value. The reverse current value 1.33A must be added, which is the displayed value of the positive current, namely 8.33A. After electroplating for 3min, it shall be transferred to DC electroplating process immediately.
Direct Current Electroplating
The current density of DC electroplating is the same as above. Take 2.0A/dm2, the plating area of 5 drill bits is 3.5dm2, the required current value is 7.0A, and the average value is 1.40A/piece. The DC pre plating time is 20~30min, and then the diamond composite plating can be started. After entering the diamond composite DC electroplating stage, the electroplating process and procedure are the same as those of nickel cobalt matrix drill bits.
In the process of direct current electroplating, how to stabilize the composition of electroplating solution and process parameters has become a key problem to ensure the quality of drill bits.
(1) Effectively prevent Fe2+ oxidation. To prevent the oxidation of Fe2+ to Fe3+, the temperature, pH value and reasonable current density of the electroplating solution must be strictly controlled. Because the increase of temperature and pH value of electroplating solution will accelerate the oxidation of Fe2+, and the possibility of ferric hydroxide precipitation in the coating will increase. At the same time, the current density should be reasonably designed and controlled according to the temperature of the electroplating solution.
(2) Fe3+ content in the plating solution is not as low as possible, especially for electroplated diamond core drill bits. For drilling hard and dense rocks, the matrix performance of the bit should have both high hardness and certain brittleness. Only the bit/segment with hardness and brittleness can have a good diamond cutting edge and obtain good drilling effect and economic and technical indicators. Therefore, the electroplating solution is required to contain a certain amount of Fe3+ to ensure that the bit matrix has high hardness and ideal brittleness.
Treatment After Electroplating
After the qualified electroplated drill bit is out of the groove, it shall be immediately washed with clean water and immersed in the alkaline solution for about half an hour to neutralize the residual acid, so as to prevent oxidation of the drill bit after it is out of the groove and affect the quality of the drill bit.
At present, there are different views on whether the plated parts soaked in alkali solution should be dehydrogenated. Practice has proved that most of the repair parts have not been dehydrogenated, which can still ensure the quality of use. Dehydrogenation treatment is indeed beneficial to prevent the coating from falling off, especially for those plated parts with complex stress, it is better to carry out dehydrogenation treatment.
Dehydrogenation treatment has advantages and disadvantages for diamond core drill bits with asymmetric AC-DC electroplating. The high temperature and long time of dehydrogenation treatment will reduce the hardness and brittleness of the matrix, which is not conducive to diamond cutting, and will reduce the drilling effect for drilling hard and dense rock formations. Without dehydrogenation treatment, the drill bit is subject to complex alternating stress at the bottom of the borehole, which may cause the hard and brittle drill bit matrix to fall off and fall off quickly. Therefore, the dehydrogenation treatment process should be different from the electroplated nickel cobalt matrix bit. The temperature of dehydrogenation treatment can be lower and the treatment time can be shortened. The specific parameters shall be determined according to the rock properties and drilling requirements after comprehensive consideration.
The bright nickel plating process should be adopted for the decoration of electroplated diamond core drill bits, which is not only simple in technology and beautiful in appearance, but also can effectively prevent the core drill bit from continuing oxidation, so as to maintain the quality of the core drill bit.
Test and Analysis of Electroplated Core Drill Bit with Iron Matrix
The components of the electroplating solution of the electroplated diamond core drill bit with iron matrix used in the test are: ferrous chloride 380g/L, ammonium chloride 25g/L, manganese dichloride 10g/L, acid barrier 10g/L. The pH value is 2.0~3.0, the solution temperature is 35~40 ℃, and the current density is 1.8A/dm2. The diamond concentration is about 95%, the diamond particle size is 45/50# accounting for 70% and 50/60# accounting for 30%.
The stone sample drilled in the test is granite gneiss with drillability of grade 9, medium fine grain size. The drill size is Φ41/27mm, the weight on bit is 4kN during indoor test, and the drill speed is 400rpm. See the table below for test drilling results.
| Core Drill Bit No. | Number of Boreholes(pcs) | Drilling Depth(m) | Cumulative Time(h) | Efficiency(m/h) | Segment Consumption Height(mm) | Estimated Service Life(m) |
|---|---|---|---|---|---|---|
| No. 1# | 12 | 10.20 | 5.10 | 2.00 | 1.20 | 42.50 |
| No. 2# | 12 | 10.20 | 5.50 | 1.85 | 1.10 | 46.36 |
| Average | 12 | 10.20 | 5.30 | 1.92 | 1.15 | 44.43 |
Note:
- ① The stone sample is a 0.9m3 cube. Because the stone sample is not very regular, the drilling depth is calculated as 0.85m/piece.
- ② The expected life is calculated based on the working layer with a bit height of 5mm.
In the drilling test, the diamond core drill bit was running very unevenly, and no segments breakage and block falling were found. During the drilling process, no diamond grains and shedding were found through tracking observation, which indicates that the iron matrix has a high firmness for diamond embedding. At present, the electroplated diamond core drill bit with iron matrix has been popularized and applied. All these indicate that the electroplated iron base material can be completely used as the matrix of the electroplated iron based diamond core drill bit, and the electroplated iron based diamond core drill bit is a new type of drill bit with high performance.